100 4 ratings well we know that Barium ions are having. One molecule of barium phosphate contains three atoms of barium two atoms of.

How To Write The Formula For Barium Phosphate Youtube
What is the correct formula for the formed compound.
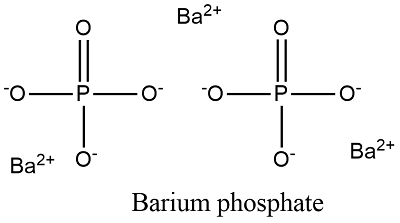
. The combination of Ba2 and PO43- would result in the formula Ba3PO42. This compound is a water-soluble salt of ammonia and aqueous ammonium chloride is slightly acidic. Barium phosphate Ba3 PO42 - PubChem.
Barium phosphate is formed from the Ba2 cation and the PO34 anion. View the full answer. Iron II phosphate c.
It is denoted by the symbol NH4Cl and is in solid crystalline form in nature. The chemical or molecular formula of Barium phosphate is Ba 3 PO 4 2. This problem has been solved.
What is the correct formula for this compound. Which has a slight odour of acetic acid. Ba3PO42 Made from BariumBa2 and PhosphatePO4-3 ions.
Chemical formula for barium phosphate. Polyatomic ions are given in the hints to the first common and in Table 35 on page 91 of Tro Select one. This is the best answer based on feedback and ratings.
Barium phosphate tribasic is colourless solid which usually occurs in its powder form. Because barium is classified as a Group 2 element we know that it produces 2 ions. What will be the formula of bariums phosphate.
Iron III phosphate b. So barium phosphate would be Ba3PO42. Barium has a valence of 2 and the phosphate radical PO4 has a valence of -3 so 32 2-3 0 as required for a compond.
Provide your answer below. What is the correct formula for this compound. Barium phosphate is formed from the Ba2 cation and the PO34 anion.
Within the formula the symbol Ba represents barium P represents phosphorus and O represents oxygen. Provide your answer below. The numbers in the chemical formula Ba3 PO42 indicate the number of atoms of each element in the compound.
Question 27 What is the correct name of Fe 3 PO 4 2. Describe the Properties of lonic Compounds Question Barium phosphate is formed from the Ba2 cation and the PO anion. Ammonium chloride also known as Sal ammoniac is a compound of ammonia NH3 and chlorine Cl.
P2O5 3BaO Ba3PO42 Barium Phosphate. It does not dissolve in water but readily dissolves in acidic liquid solutions. The chemical formula for barium phosphate is Ba3 PO42.
What is the correct formula for an ionic compound that contains barium ions and phosphate ions. See the answer See the answer done loading. What is the correct formula for this compound.
By Sushil Khadka. The phosphate anion is PO4 3-. If you happen to have a chart of common polyatomic ions you may see that phosphate is represented by the symbol PO4 and has a charge of 3.
The barium cation is Ba2.
Barium Phosphate Ba3 Po4 2 Pubchem

0 Comments